Towards an Innovative and Novel Therapy for Treating Neurological Conditions
The latest advances in this field indicate that combining multiple targets is currently essential to effectively treat these highly complex disorders.
Thus, the device's photonic technology acts synergistically at the mitochondrial level, while considering the impact of local and peripheral inflammation in diseases.
The RGn device is also designed to act on the brain and/or the gut, in accordance with the latest studies demonstrating the involvement of the gut microbiota in inflammatory processes.

Tri-photonic Stimulation
REGEnLIFE uses a combination of photobiomodulation associated with magnetic fields called “Tri-photonic Stimulation” (3 complementary light sources with precise wavelengths between red and infrared, associated with a static magnetic field).
This technology is innovative in a field where research and discoveries are made every year to provide increasingly precise answers to the mechanisms involved.
These results constantly outline areas for improvement for optimizing the parameters on which the device’s performance depends.
“REGEnLIFE RESEARCH”, an Internationally Renowned Multidisciplinary Team
REGEnLIFE’s strength lies in having brought together doctors and engineers who, combining their knowledge and skills within our research & development department, thus acquire the ability to precisely master all these parameters: wavelengths, photon direction, “intensity”, fluence of the generated light…(+ details)
It is this particular attention paid to the constitution of our research & development department “REGEnLIFE RESEARCH”, in its composition and the resources allocated to it, that has made possible the considerable advances we have achieved in this field.
R&D efforts have led REGEnLIFE to develop a comprehensive patent portfolio:
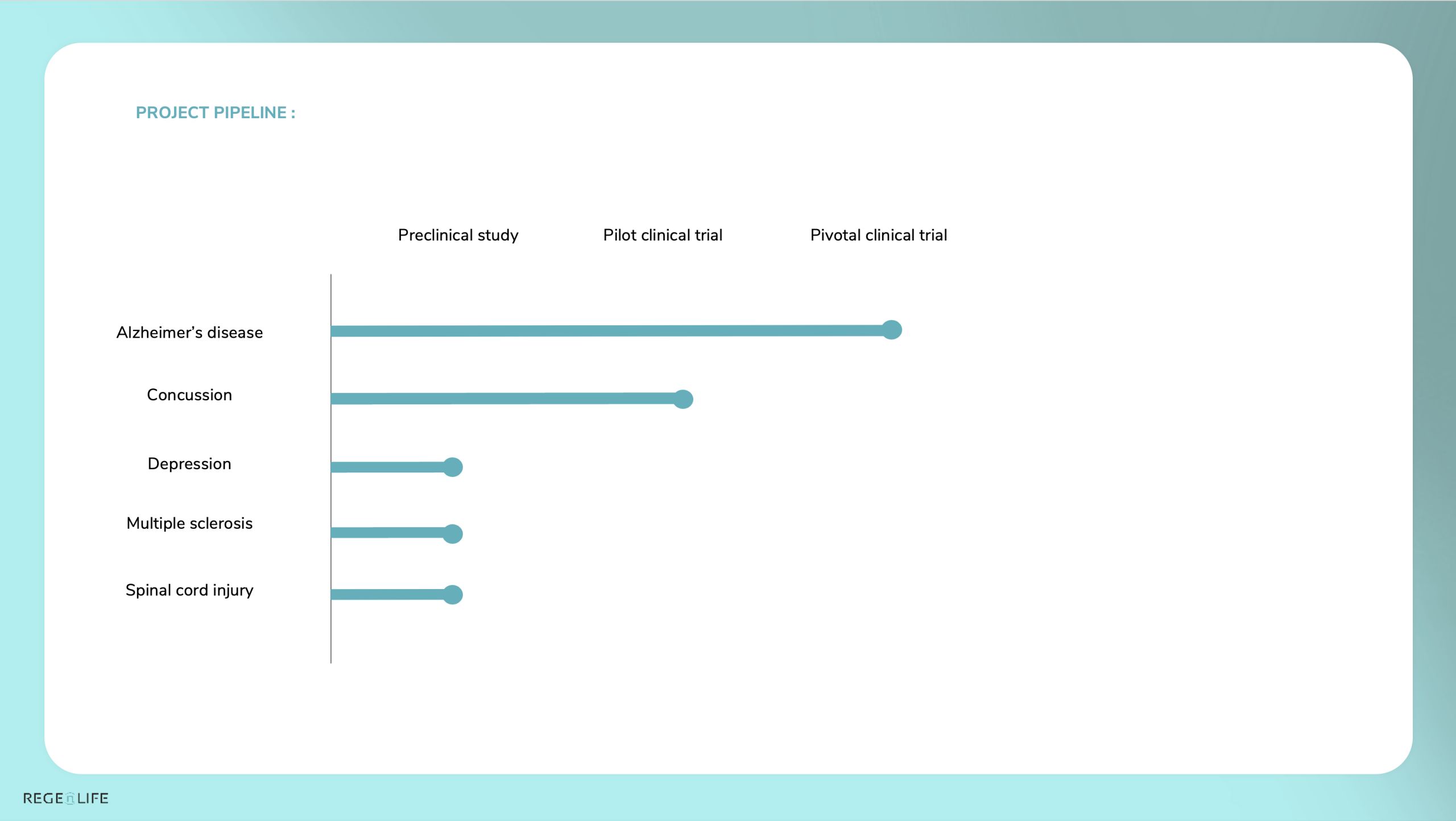
Today, REGEnLIFE is Finalizing:

ALZHEIMER’S DISEASE
• After obtaining positive results in an animal model in a preclinical study, a pilot clinical study was conducted and demonstrated the device’s safety. Positive trends were observed regarding the technology’s therapeutic efficacy.
• A pivotal study (phase 3) is underway to confirm the device’s efficacy and demonstrate a cognitive benefit from REGEnLIFE technology.

CONCUSSION
• Publication of our RECOVERY pilot study on concussion
• Preparation of the European market authorization application (CE medical marking)
OTHER PROJECTS
• Preclinical work has been finalized, making it possible to consider a transition to clinical trials for depression and multiple sclerosis.



