ALZHEIMER’S DISEASE
A progressive neurodegenerative pathology affecting the brain, Alzheimer's disease mainly manifests itself through memory impairment, but also impacts language, reasoning, behavior and autonomy. A source of morbidity, disability and mortality, it has major social and economic consequences, with an estimated annual global cost of $1,300 billion.

Pathophysiology of Alzheimer’s disease
Alzheimer’s disease is a progressive neurodegenerative disorder, with neuronal degeneration in the hippocampus gradually spreading to the whole brain.
The pathophysiology of Alzheimer’s disease is based on a complex cascade of cellular and biochemical events. It is characterized by the extraneuronal accumulation of β-amyloid proteins in the form of plaques, and the intraneuronal accumulation of hyperphosphorylated tau proteins in the form of neurofibrillary degeneration. These plaques and neurofibrillary degenerations lead to progressive neuronal loss and synaptic degeneration.
The very progressive development of these two specific lesions is associated with non-specific pathological mechanisms accelerating neuronal suffering and death: oxidative stress, neuroinflammation and mitochondrial damage.
Mitochondria are intracellular organelles that provide the energy essential for neuron function. In addition, oxidative stress, when not sufficiently controlled by the immune system, becomes powerfully neurotoxic. In addition, a growing body of scientific work underlines the importance of neuroinflammation and the cells surrounding neurons – astrocytes and microglia – in the pathophysiological cascade of Alzheimer’s disease. Finally, changes in the composition of the intestinal microbiome may also play a role in Alzheimer’s disease.
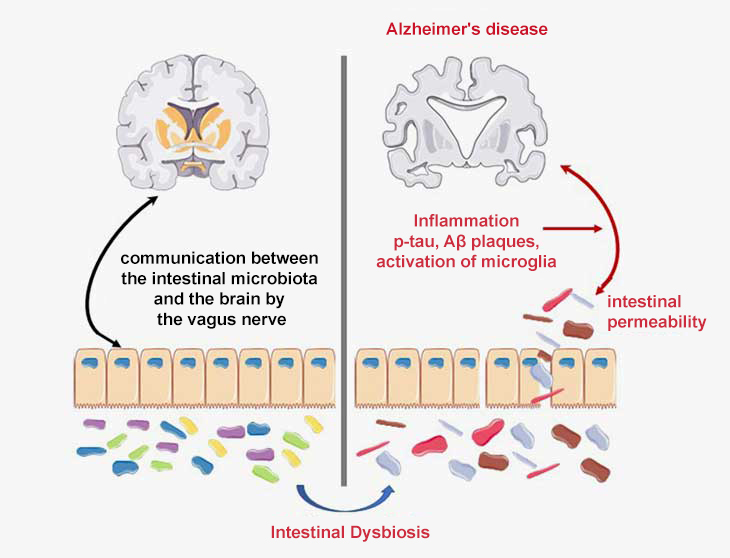
The role of the brain-gut-microbiota axis in Alzheimer’s disease
The immune system is responsible for bidirectional communication between the gut and the brain. Numerous studies have demonstrated the involvement of disruptions to the brain-microbiota-gut axis in the pathophysiology of neurological conditions such as Alzheimer’s disease (Doifode et al., 2021).
Brain and photobiomodulation
Photobiomodulation therapy can benefit a wide range of brain disorders by targeting mitochondria, thereby reducing inflammation and apoptosis, and increasing cerebral blood flow and oxygenation.
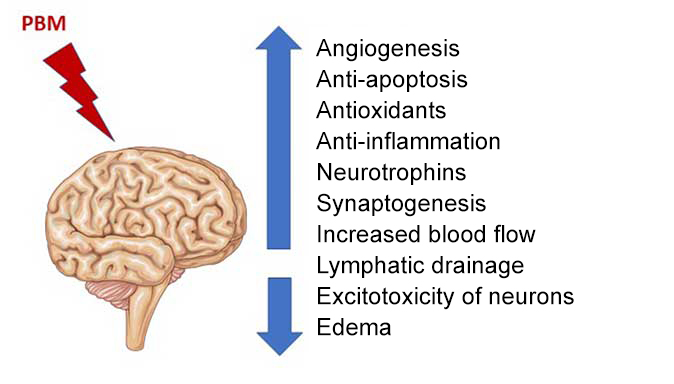
The therapeutic contribution of PBM could be significant for many neurological pathologies.
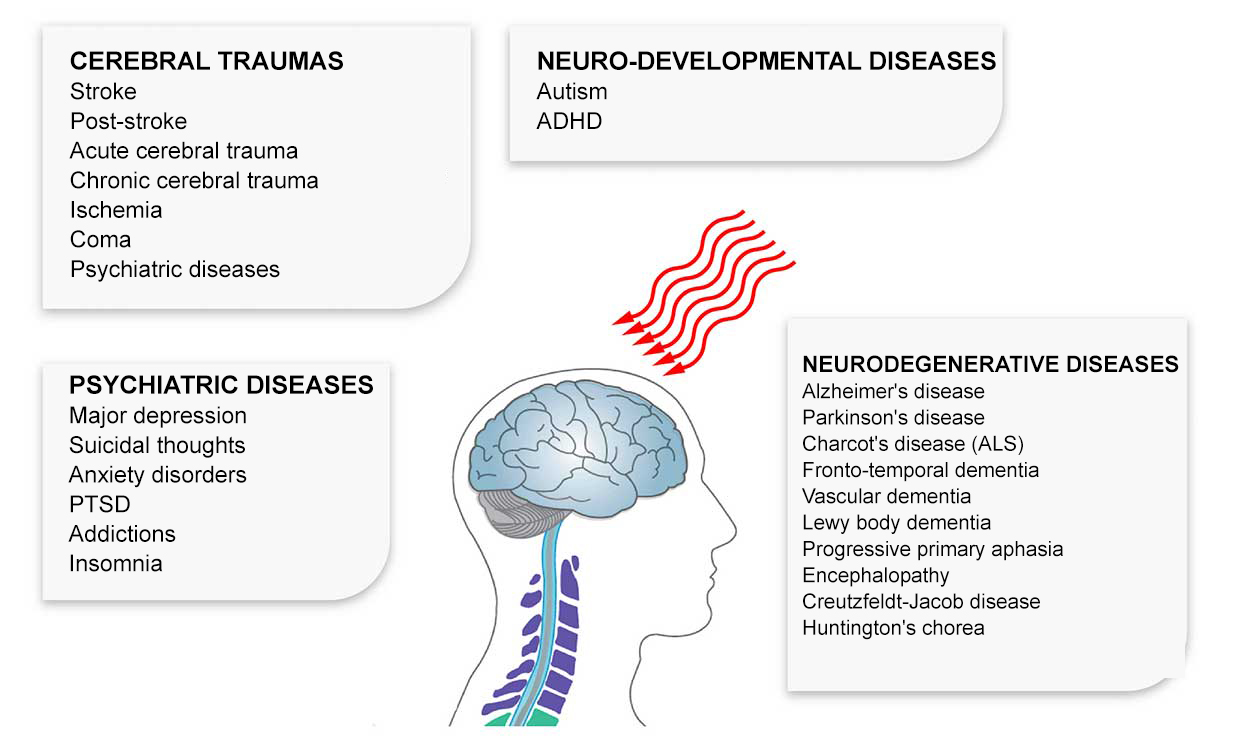
Photobiomodulation is now emerging as a potential treatment for Alzheimer’s-type dementia.

TheЕssai clinique pivot LIGHT4LIFE (NCT05926011): underway to evaluate REGEnLIFE technology onAlzheimer’s patients
RGn600, a new version of REGEnLIFE technology, is currently being evaluated in a multi-center, double-blind, randomized clinical trial in partnership with Toulouse University Hospital’s Gérontopôle and other investigating centers.

Pilot clinical trial ofREGEnLIFE’sphotomedical technologyfor the treatment ofAlzheimer’s disease
RGn was shown to be safe and well tolerated in a pilot clinical study of Alzheimer’s disease.

Preclinical studiesby REGEnLIFE
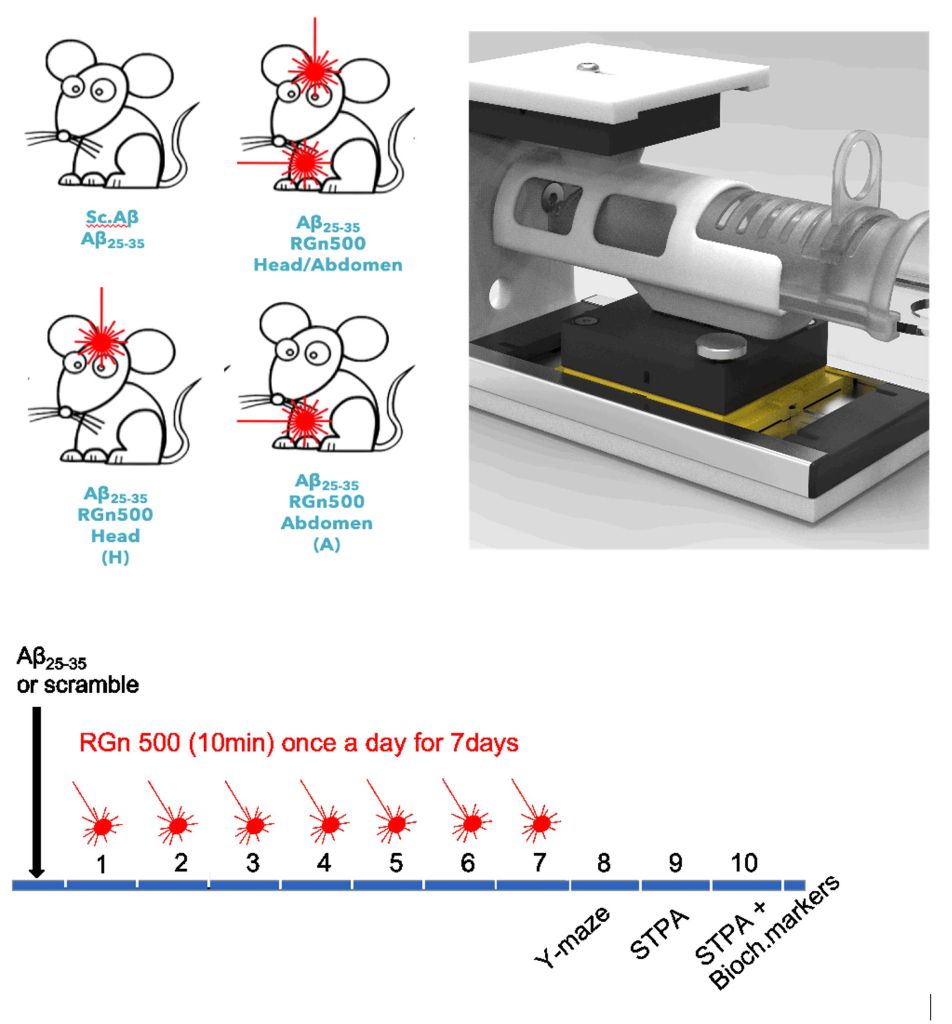
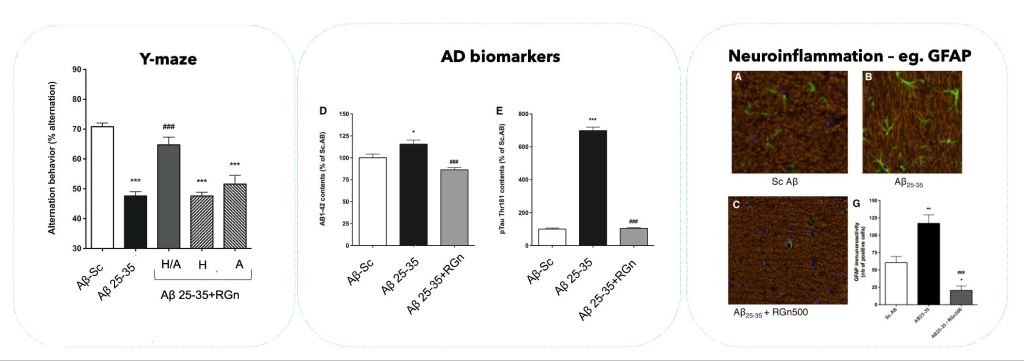
In preclinical studies, the RGn500 prototype has been shown to normalize memory deficits and biochemical changes in the Aβ25-35 mouse model, mimicking the pathological features of Alzheimer’s disease.
The Еssai clinique pivot Light4Life: underway to evaluate REGEnLIFE technology on Alzheimer’s patients
RGn600, a new version of REGEnLIFE technology, is currently being evaluated in a multi-center, double-blind, randomized clinical trial in partnership with Toulouse University Hospital’s Gérontopôle and other investigating centers.
Context
Alzheimer’s disease (AD) represents a serious public health problem, for which no clearly effective treatment is currently available.
The “REGEnLIFE RGn600” is a photobiomodulation (PBM) medical device targeting the brain and gut. Results from an initial pilot clinical trial demonstrated safety and patient compliance, and revealed efficacy trends in 53 patients with mild-to-moderate Alzheimer’s disease treated with PBM therapy (Blivet et al., A randomized, double-blind, and sham-controlled trial of an innovative brain-gut photobiomodulation therapy: safety and patient compliance. J Alzheimer’s Dis. 2022, 90: 2). This study provided valuable information for the design of our pivotal therapeutic trial, which is currently evaluating the cognitive benefits of photobiomodulation therapy in a larger sample of Alzheimer’s disease patients.
Objectives
The primary endpoint of this pivotal clinical trial is the evolution of patients’ cognition after 26 weeks of PBM therapy, measured by the ADAS-cog score. Neuropsychological function, autonomy, overall clinical response, quality of life, blood and fecal markers and medico-economic value are studied as secondary endpoints. The safety of RGn600 treatment was also evaluated.
Methods
The RGn600 is a non-invasive medical device, manufactured by REGEnLIFE, which comes in the form of a helmet and abdominal belt combining PBM technology from red to near-infrared wavelengths and static magnetic stimulation.
A pivotal multicenter, double-blind, randomized, controlled clinical trial has been initiated in July 2023 at the Toulouse University Hospital Gérontopole. A total of 108 patients with NIA-AA diagnostic criteria for AD will be included and randomized into a treatment group (n=54) and a sham group (n=54). They will receive 84 sessions of 20 min PBM therapy over 26 weeks.
Results
The first patient visit took place in July 2023. Statistical analysis will be performed in the full analysis set, the per-protocol populations and the treated set.
Conclusion
REGEnLIFE RGn600 brain-gut PBM therapy, as a treatment, could potentially offer a safe, well-tolerated method, with medical and economic benefits, to treat patients with mild to moderate AD. The design of this study, named LIGHT4LIFE, evaluating such an innovative medical device for the treatment of AD is detailed (NCT05926011). The advantages and limitations of this clinical trial will be discussed.
Pilot clinical trial of REGEnLIFE’s photomedical technology for the treatment of Alzheimer’s disease
RGn was shown to be safe and well tolerated in a pilot clinical study of Alzheimer’s disease.
In March 2021, REGEnLIFE announced encouraging initial results from its first pilot clinical study evaluating its medical device on Alzheimer’s disease. They were presented at the 15th International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD 2021) from March 9 to 14, by Professor Jacques Touchon, scientific advisor on the clinical trial.
REGEnLIFE’s innovative, non-invasive technology, which is the subject of this therapeutic trial, is based on a photobiomodulation technique designed to target the brain and gut, using a helmet and abdominal breastplate. This novel medical device in photo-medicine, called RGn, aims to stimulate cells in the brain and intestine and regulate inflammation, so as to improve cognitive functioning and behavior. It targets inflammation on the brain-gut axis, which is thought to be involved in the development of Alzheimer’s disease and other neurological disorders.
Design of REGEnLIFE’s pilot clinical study in Alzheimer’s disease:
Pilot study evaluating the safety and efficacy of a photobiomodulation prototype, RGn, developed by REGEnLIFE, on cognitive performance in patients with mild to moderate Alzheimer’s disease. (NCT03672474)
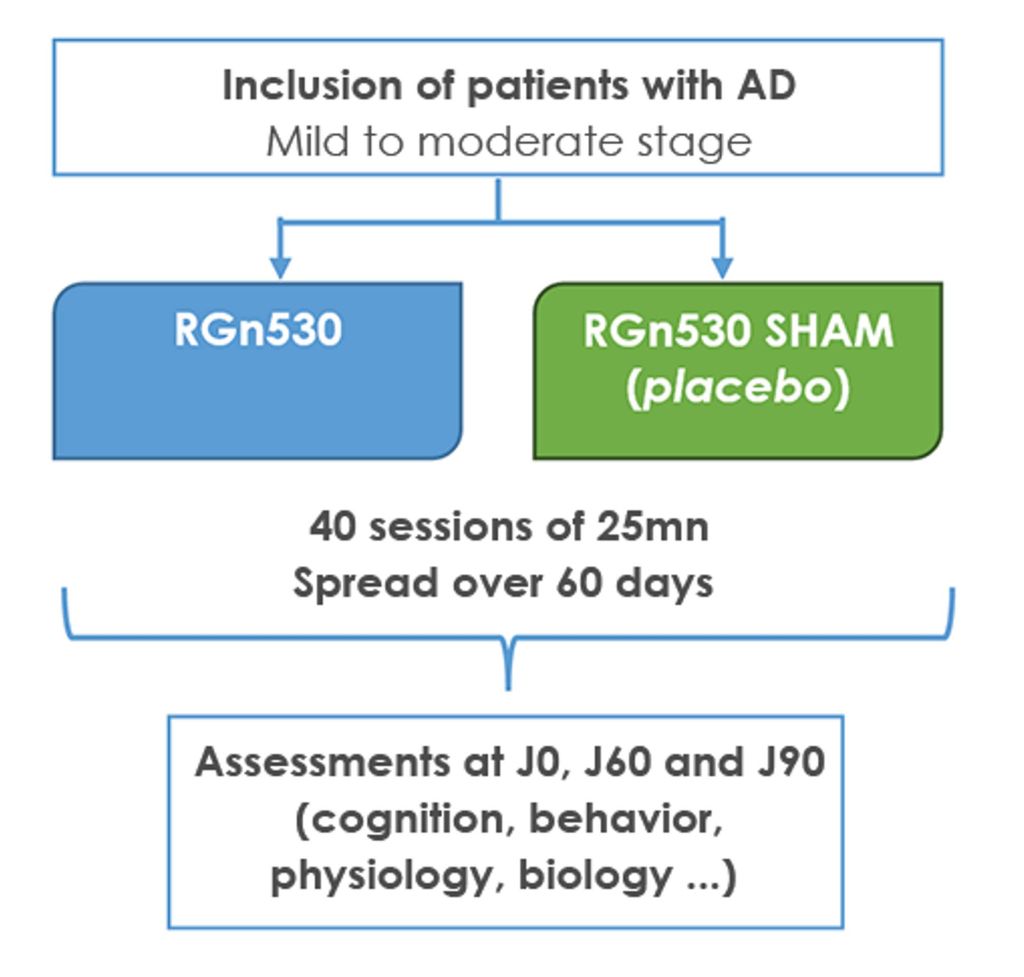
The trial involved adult volunteers aged 55 to 85 with mild to moderate Alzheimer’s disease. Equipped with a helmet and a photobiomodulation abdominal belt, these patients benefited from 40 25-minute sessions spread over two months, and were evaluated by a series of tests throughout the study and up to one month after stopping treatment. This double-blind, randomized, single-center, placebo-controlled clinical trial began in 2018 and ended prematurely in 2020 due to the COVID-19 pandemic. Of the 64 planned, 53 patients were randomized into two groups (treated and placebo) and 43 patients were able to benefit from treatment for the planned duration.
- Treatment group shows trends towards cognitive improvement, executive function, language comprehension and verbal memory compared to placebo group
- REGEnLIFE’s technology has been shown to be safe and well tolerated by treated patients
- These results open up new prospects for the development of this novel device for the prevention and treatment of neurological disorders using both cerebral and intestinal stimulation.
The primary efficacy endpoint was measured by the change in ADAS-Cog (Alzheimer’s Disease Assessment Scale) total score between inclusion and two months of treatment. In terms of safety, the REGEnLIFE RGn device was found to be safe, with no serious adverse events reported. Compliance with treatment sessions was very good for the vast majority of patients (92%), confirming the device’s good tolerance. Although the primary efficacy criterion was not met in statistical terms, there was a clear trend towards improvement in a range of cognitive functions. The results of this pilot study showed that REGEnLIFE technology is safe and well tolerated by patients. These very encouraging safety and efficacy results need to be confirmed in a pivotal or phase III clinical trial.
“To be effective, the therapeutic strategy for Alzheimer’s disease needs to address several targets. Drug treatments targeting the two proteins characteristic of the Alzheimer process (beta-amyloid and tau proteins) need to be complemented by other therapies targeting less specific but very important mechanisms in the physiopathological cascade, such as inflammation and oxidative stress. The photobiomodulation technology developed by REGEnLIFE acts far upstream on this cascade (mitochondria, inflammation, oxidative stress). It could be the non-medicinal complement to the therapeutic strategy of the future. What’s more, REGEnLIFE’s technology enables us to act on both the brain and the intestine, a considerable advantage given the importance of the brain-intestine axis and the microbiota in neurological disorders”, adds Professor Jacques Touchon, neurologist and psychiatrist, scientific advisor on this clinical trial.
In a nutshell:
- This pilot study proved the feasibility and tolerability of REGEnLIFE’s RGn device.
- The RGn device is also safe and enables non-invasive treatment.
- Patients were highly compliant with RGn treatment.
- This study showed trends in cognitive efficacy despite the reduced number of patients due to the premature termination of the study because of COVID-19.
- This study also provides valuable insights for the design of the next phase of clinical trials aimed at demonstrating, on a larger scale, the cognitive benefit of RGn treatment.
References :
Blivet et al, Safety and feasibility of the RGn, an innovative brain-gut photobiomodulation device, in mild-to-moderate Alzheimer’s Disease patients: a randomized, double-blind and sham-controlled trial, Oral communication AD/PD 2021.
Karol Kowalski and Agata Mulak – Brain-Gut-Microbiota Axis in Alzheimer’s Disease, January 2019
Preclinical studies conducted by REGEnLIFE
In preclinical studies, the RGn500 prototype has been shown to normalize memory deficits and biochemical changes in the Aβ25-35 mouse model, mimicking the pathological features of AD.

In preclinical studies, the RGn500 prototype has been shown to normalize memory deficits and biochemical changes in the Aβ25-35 mouse model, mimicking the pathological features of AD.
Interestingly, this neuroprotective effect, in the configurations studied, was only observed when both the head and abdomen were exposed (but not the head only or the abdomen only), suggesting that several mechanisms are involved, including direct activation of cellular chromophores at the neuronal level and indirect effects resulting from abdominal exposure. It also suggests that double PBM exposure, on the head and abdomen, would increase therapeutic efficacy.