ALZHEIMER’S DISEASE
A progressive neurodegenerative pathology affecting the brain, Alzheimer's disease mainly manifests itself through memory impairment, but also impacts language, reasoning, behavior and autonomy. A source of morbidity, disability and mortality, it has major social and economic consequences, with an estimated annual global cost of $1,300 billion.

Treating Alzheimer’s disease
Despite advances in research, there is no treatment that can prevent, cure or halt the progression of Alzheimer’s disease. Recent advances in treatments (pharmacological and non-pharmacological) targeting the pathophysiology of the disease represent a promising departure from the classic symptomatic approach.
The first Alzheimer’s therapies on the market were only symptomatic. From 2003 to 2021, despite numerous clinical trials, no new treatment for Alzheimer’s disease had been approved.
The arrival in 2021 of treatments targeting the pathophysiology of the disease has marked a turning point. Indeed, the anti-amyloid monoclonal antibodies recently approved by the FDA (USA) and the EMA (Europe) are giving fresh impetus to the hope of a therapy to attenuate cognitive decline. These treatments aim to reduce or eliminate neuron-destroying β-amyloid protein deposits in the brain. However, concerns remain as to their long-term efficacy and safety. Other new pharmacological approaches (tau aggregation inhibitors, immunotherapies, etc.) are still being evaluated.
Given the complexity of Alzheimer’s disease, the need to integrate combined drug and non-drug therapeutic strategies, targeting a variety of pathological processes, is increasingly recognized. In this context, non-pharmacological interventions are gaining in importance as potential key elements of a comprehensive combined therapeutic plan.
Neurotechnologies and new forms of neurostimulation based on invasive (implantable) and non-invasive (transcranial) applications are very promising. These new forms of stimulation can be electrical (tES – transcranial electrical stimulation), ultrasonic, magnetic (TMS – transcranial magnetic stimulation) or photonic (transcranial photobiomodulation).
In the field of neurotechnology and photomedicine, REGEnLIFE is developing an innovative treatment with both preventive and curative action: Tri-Photonic Stimulation. This is a non-invasive neuro-intestinal application acting essentially on inflammation, and based on photonic emission combined with a magnetic field. This technological and therapeutic positioning is currently unprecedented.
The treatment developed by REGEnLIFE will thus be able to meet the future challenges of combined treatments for Alzheimer’s disease.
Key figures
In 2023, REGEnLIFE, in conjunction with the Gérontopôle du CHU de Toulouse, a pivotal clinical trial on Alzheimer’s disease(LIGHT4LIFE). to confirm the efficacy of its technology over a longer period of time and on a larger number of patients. Initial results are expected by the end of 2026.
Presentation of work in progress for each indication:

TheЕssai clinique pivot LIGHT4LIFE (NCT05926011): underway to evaluate REGEnLIFE technology onAlzheimer’s patients
RGn600, a new version of REGEnLIFE technology, is currently being evaluated in a multi-center, double-blind, randomized clinical trial in partnership with Toulouse University Hospital’s Gérontopôle and other investigating centers.

Pilot clinical trial ofREGEnLIFE’sphotomedical technologyfor the treatment ofAlzheimer’s disease
RGn was shown to be safe and well tolerated in a pilot clinical study of Alzheimer’s disease.

Preclinical studiesby REGEnLIFE
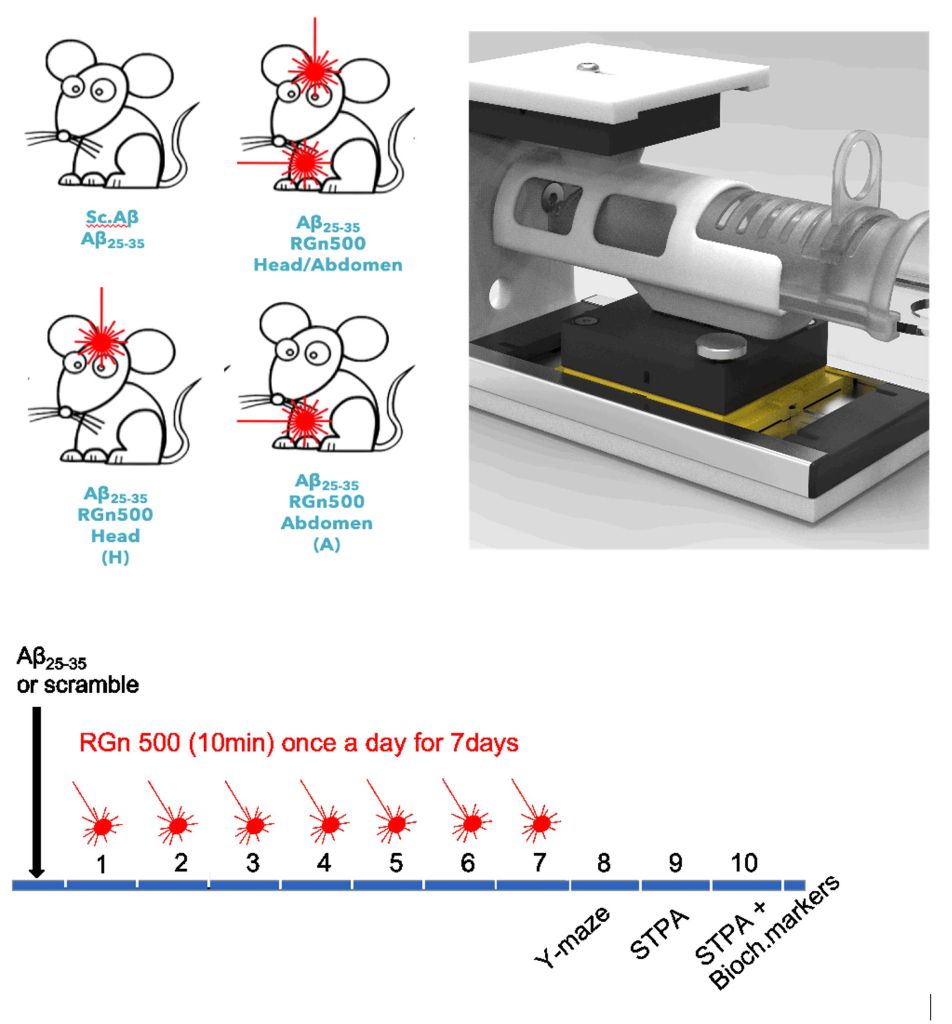
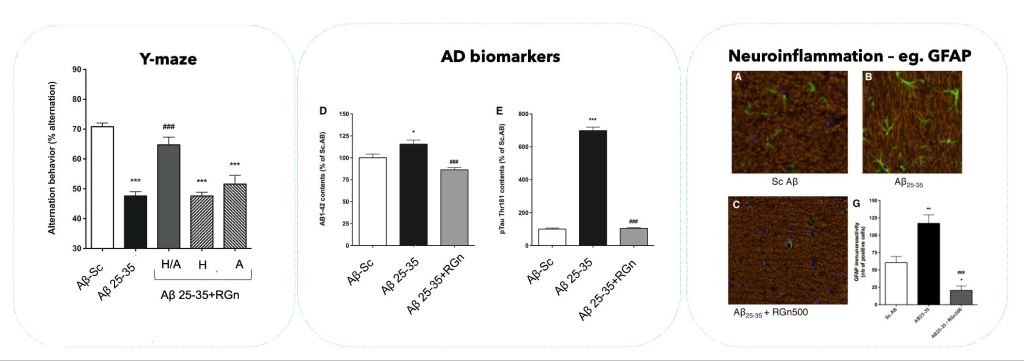
In preclinical studies, the RGn500 prototype has been shown to normalize memory deficits and biochemical changes in the Aβ25-35 mouse model, mimicking the pathological features of Alzheimer’s disease.
The Еssai clinique pivot Light4Life: underway to evaluate REGEnLIFE technology on Alzheimer’s patients
RGn600, a new version of REGEnLIFE technology, is currently being evaluated in a multi-center, double-blind, randomized clinical trial in partnership with Toulouse University Hospital’s Gérontopôle and other investigating centers.
Context
Alzheimer’s disease (AD) represents a serious public health problem, for which no clearly effective treatment is currently available.
The “REGEnLIFE RGn600” is a photobiomodulation (PBM) medical device targeting the brain and gut. Results from an initial pilot clinical trial demonstrated safety and patient compliance, and revealed efficacy trends in 53 patients with mild-to-moderate Alzheimer’s disease treated with PBM therapy (Blivet et al., A randomized, double-blind, and sham-controlled trial of an innovative brain-gut photobiomodulation therapy: safety and patient compliance. J Alzheimer’s Dis. 2022, 90: 2). This study provided valuable information for the design of our pivotal therapeutic trial, which is currently evaluating the cognitive benefits of photobiomodulation therapy in a larger sample of Alzheimer’s disease patients.
Objectives
The primary endpoint of this pivotal clinical trial is the evolution of patients’ cognition after 26 weeks of PBM therapy, measured by the ADAS-cog score. Neuropsychological function, autonomy, overall clinical response, quality of life, blood and fecal markers and medico-economic value are studied as secondary endpoints. The safety of RGn600 treatment was also evaluated.
Methods
The RGn600 is a non-invasive medical device, manufactured by REGEnLIFE, which comes in the form of a helmet and abdominal belt combining PBM technology from red to near-infrared wavelengths and static magnetic stimulation.
A pivotal multicenter, double-blind, randomized, controlled clinical trial has been initiated in July 2023 at the Toulouse University Hospital Gérontopole. A total of 108 patients with NIA-AA diagnostic criteria for AD will be included and randomized into a treatment group (n=54) and a sham group (n=54). They will receive 84 sessions of 20 min PBM therapy over 26 weeks.
Results
The first patient visit took place in July 2023. Statistical analysis will be performed in the full analysis set, the per-protocol populations and the treated set.
Conclusion
REGEnLIFE RGn600 brain-gut PBM therapy, as a treatment, could potentially offer a safe, well-tolerated method, with medical and economic benefits, to treat patients with mild to moderate AD. The design of this study, named LIGHT4LIFE, evaluating such an innovative medical device for the treatment of AD is detailed (NCT05926011). The advantages and limitations of this clinical trial will be discussed.
Castres, France, 81100
Patient recruitment in progress
CHIC Castres Mazamet Site Autan
Contact :
Marie-Noëlle Cufi, Geriatrician
marie-noelle.cufi@chic-cm.fr
Lavaur, France, 81500
Patient recruitment in progress
CH Lavaur
Contact :
Céline Guillemaud, Geriatrician
c.guillemaud@ch-lavaur.fr
Paris, France, 75010
Patient recruitment in progress
Hôpital Lariboisière, AP-HP
Contact :
Claire PAQUET, Neurologist
claire.paquet@aphp.fr
Paris, France, 75013
Patient recruitment in progress
Hôpital Broca, AP-HP
Contact :
Marie Laure SEUX, Geriatrician
marie-laure.seux@aphp.fr
Toulouse, France, 31 000
Patient recruitment in progress
CHU de Toulouse-Gerontopole
Contact :
Julien DELRIEU, Neurologist-Geriatrician +33 5 61 77 70 58
delrieu.j@chu-toulouse.fr
Pilot clinical trial of REGEnLIFE’s photomedical technology for the treatment of Alzheimer’s disease
RGn was shown to be safe and well tolerated in a pilot clinical study of Alzheimer’s disease.
In March 2021, REGEnLIFE announced encouraging initial results from its first pilot clinical study evaluating its medical device on Alzheimer’s disease. They were presented at the 15th International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD 2021) from March 9 to 14, by Professor Jacques Touchon, scientific advisor on the clinical trial.
REGEnLIFE’s innovative, non-invasive technology, which is the subject of this therapeutic trial, is based on a photobiomodulation technique designed to target the brain and gut, using a helmet and abdominal breastplate. This novel medical device in photo-medicine, called RGn, aims to stimulate cells in the brain and intestine and regulate inflammation, so as to improve cognitive functioning and behavior. It targets inflammation on the brain-gut axis, which is thought to be involved in the development of Alzheimer’s disease and other neurological disorders.
Design of REGEnLIFE’s pilot clinical study in Alzheimer’s disease:
Pilot study evaluating the safety and efficacy of a photobiomodulation prototype, RGn, developed by REGEnLIFE, on cognitive performance in patients with mild to moderate Alzheimer’s disease. (NCT03672474)
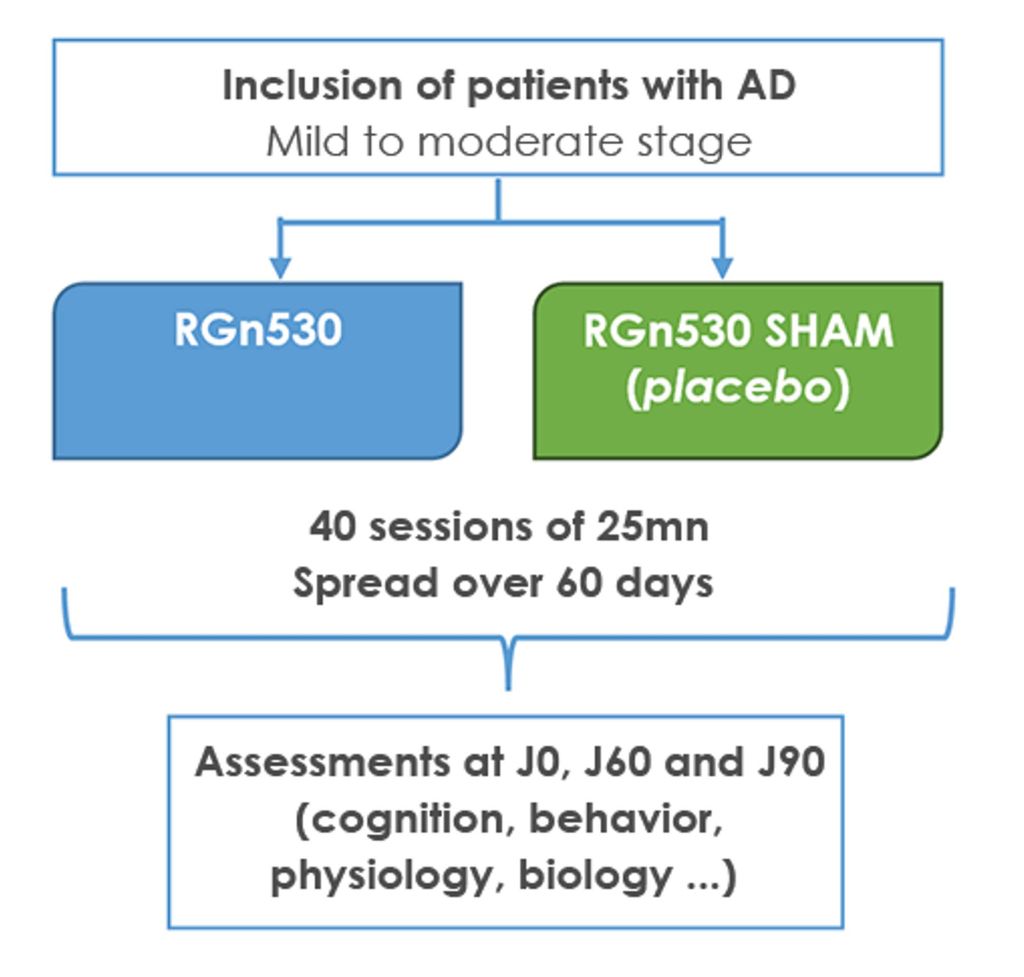
The trial involved adult volunteers aged 55 to 85 with mild to moderate Alzheimer’s disease. Equipped with a helmet and a photobiomodulation abdominal belt, these patients benefited from 40 25-minute sessions spread over two months, and were evaluated by a series of tests throughout the study and up to one month after stopping treatment. This double-blind, randomized, single-center, placebo-controlled clinical trial began in 2018 and ended prematurely in 2020 due to the COVID-19 pandemic. Of the 64 planned, 53 patients were randomized into two groups (treated and placebo) and 43 patients were able to benefit from treatment for the planned duration.
- Treatment group shows trends towards cognitive improvement, executive function, language comprehension and verbal memory compared to placebo group
- REGEnLIFE’s technology has been shown to be safe and well tolerated by treated patients
- These results open up new prospects for the development of this novel device for the prevention and treatment of neurological disorders using both cerebral and intestinal stimulation.
The primary efficacy endpoint was measured by the change in ADAS-Cog (Alzheimer’s Disease Assessment Scale) total score between inclusion and two months of treatment. In terms of safety, the REGEnLIFE RGn device was found to be safe, with no serious adverse events reported. Compliance with treatment sessions was very good for the vast majority of patients (92%), confirming the device’s good tolerance. Although the primary efficacy criterion was not met in statistical terms, there was a clear trend towards improvement in a range of cognitive functions. The results of this pilot study showed that REGEnLIFE technology is safe and well tolerated by patients. These very encouraging safety and efficacy results need to be confirmed in a pivotal or phase III clinical trial.
“To be effective, the therapeutic strategy for Alzheimer’s disease needs to address several targets. Drug treatments targeting the two proteins characteristic of the Alzheimer process (beta-amyloid and tau proteins) need to be complemented by other therapies targeting less specific but very important mechanisms in the physiopathological cascade, such as inflammation and oxidative stress. The photobiomodulation technology developed by REGEnLIFE acts far upstream on this cascade (mitochondria, inflammation, oxidative stress). It could be the non-medicinal complement to the therapeutic strategy of the future. What’s more, REGEnLIFE’s technology enables us to act on both the brain and the intestine, a considerable advantage given the importance of the brain-intestine axis and the microbiota in neurological disorders”, adds Professor Jacques Touchon, neurologist and psychiatrist, scientific advisor on this clinical trial.
In a nutshell:
- This pilot study proved the feasibility and tolerability of REGEnLIFE’s RGn device.
- The RGn device is also safe and enables non-invasive treatment.
- Patients were highly compliant with RGn treatment.
- This study showed trends in cognitive efficacy despite the reduced number of patients due to the premature termination of the study because of COVID-19.
- This study also provides valuable insights for the design of the next phase of clinical trials aimed at demonstrating, on a larger scale, the cognitive benefit of RGn treatment.
References :
Blivet et al, Safety and feasibility of the RGn, an innovative brain-gut photobiomodulation device, in mild-to-moderate Alzheimer’s Disease patients: a randomized, double-blind and sham-controlled trial, Oral communication AD/PD 2021.
Karol Kowalski and Agata Mulak – Brain-Gut-Microbiota Axis in Alzheimer’s Disease, January 2019
Preclinical studies conducted by REGEnLIFE
In preclinical studies, the RGn500 prototype has been shown to normalize memory deficits and biochemical changes in the Aβ25-35 mouse model, mimicking the pathological features of AD.

In preclinical studies, the RGn500 prototype has been shown to normalize memory deficits and biochemical changes in the Aβ25-35 mouse model, mimicking the pathological features of AD.
Interestingly, this neuroprotective effect, in the configurations studied, was only observed when both the head and abdomen were exposed (but not the head only or the abdomen only), suggesting that several mechanisms are involved, including direct activation of cellular chromophores at the neuronal level and indirect effects resulting from abdominal exposure. It also suggests that double PBM exposure, on the head and abdomen, would increase therapeutic efficacy.