Complex neurological pathologies, such as Alzheimer’s disease, traumatic brain injuries, multiple sclerosis, or resistant depression, represent one of the greatest current medical and societal challenges. With 3.4 billion people affected by a neurological condition during their lifetime (Lancet neurology, 2024), representing 40% of the world’s population, the need for effective therapeutic solutions is becoming urgent.
Complex neurological pathologies, such as Alzheimer’s disease, traumatic brain injuries, multiple sclerosis, or resistant depression, represent one of the greatest current medical and societal challenges. With 3.4 billion people affected by a neurological condition during their lifetime (Lancet neurology, 2024), representing 40% of the world’s population, the need for effective therapeutic solutions is becoming urgent.

Complex neurological pathologies, such as Alzheimer’s disease, traumatic brain injuries, multiple sclerosis, or resistant depression, represent one of the greatest current medical and societal challenges. With 3.4 billion people affected by a neurological condition during their lifetime (Lancet neurology, 2024), representing 40% of the world’s population, the need for effective therapeutic solutions is becoming urgent.
out of 1000
a concussion
each year
in France and
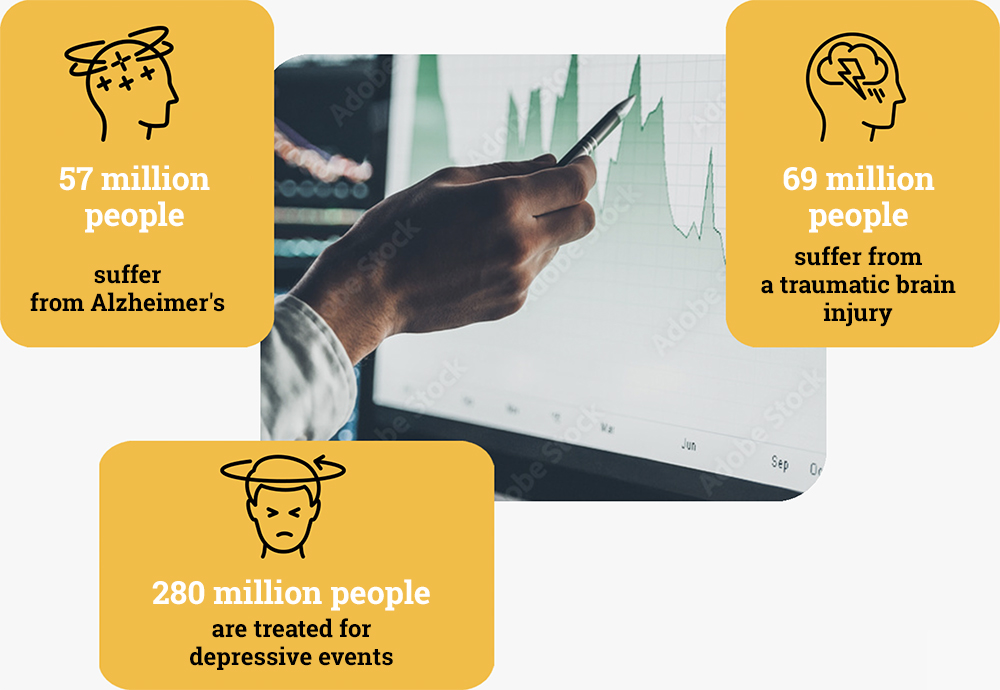
55 million
cases worldwide.

In a leading position to address these challenges, REGEnLIFE is preparing to revolutionize the treatment of neurological conditions with a pioneering, patented therapeutic solution that is close to market launch.
REGEnLIFE technology aims to activate regenerative and vital cellular repair processes. Targeted tri-photonic stimulation towards cerebral and intestinal areas promotes neuroprotection and modulates neuroinflammation to reduce symptoms and improve patients’ brain health.
The REGEnLIFE medical device takes the form of a helmet and an abdominal belt, connected to an electronic console and software-controlled. Non-invasive and painless for the patient, it allows for a dual application of neurostimulation, both cerebral and/or intestinal, depending on the pathology. The specific combination of optical components and precise wavelengths (LEDs, near-infrared lasers…) promotes optical absorption at the intracellular level, with the aim of triggering therapeutic effects. The light, precisely delivered during several 20-minute sessions, aims to activate mitochondrial function, reduce oxidative stress, and target inflammation, all key elements in the treatment of neurological diseases.
REGEnLIFE is preparing to commercialize an integrated platform, including its medical devices, software, consumables, and associated services, to dedicated centers or spaces within hospitals, EPHADs (nursing homes for dependent elderly people), retirement homes, medical practices, etc.

Why REGEnLIFE?
Exceptional market potential : The WHO estimates that the direct and indirect cost of dementia worldwide amounted to $1,300 billion in 2019, and that central nervous system therapy expenditures are expected to reach $166 billion in 2028.
Differentiating technology : REGEnLIFE’s proprietary technology combines photobiomodulation with dual cranial and intestinal application, offering unique prospects for treating neurological pathologies.
Imminent market access for the treatment of concussions : REGEnLIFE is close to commercializing, in the EU, the first photonic neurostimulation device approved in neurology.
A promising pipeline : REGEnLIFE’s therapeutic portfolio includes Alzheimer’s disease, concussion, depression, multiple sclerosis, and spinal cord injuries… with an even broader application potential. Several preclinical and clinical studies in different indications are currently underway, with initial results showing treatment efficacy, safety, very good tolerability, and excellent patient compliance.
High barriers to entry : REGEnLIFE’s know-how, its proprietary technology protected by 33 patents across 4 families, and its network of collaborations (CNRS, Université Paris-Saclay, Aix-Marseille Université, Hôpital européen Georges Pompidou, Universitat de Barcelona, Hôpitaux de Toulouse, AP-HP…), constitute major assets against competition.
Strong societal impact : a project that could transform the lives of millions of patients and their families.
We are not just developing innovative medical devices; we are building the future of brain health. With our investor partners, we can accelerate the transition from research to treatment and transform an ambitious scientific vision into clinical reality.
The REGEnLIFE medical device, based on a brain-gut application with mechanisms of action on cellular metabolism and inflammation, constitutes a therapeutic innovation under development for several neurological conditions.
Preclinical and Clinical Developments (Ongoing or Published)
- Concussions :
The RECOVERY pilot clinical trial including a cohort of 50 athletes treated less than 72 hours after the concussive impact, showed positive results (JF Chermann et al. ISSC 2023). Application for EU market authorization of the RGn device for the treatment of concussions. - Alzheimer’s Disease :
The pilot clinical trial showed no side effects and promising results regarding cognitive improvement in 43 patients over 2 months of treatment (Journal of Alzheimer’s Disease, 2022).
The multicenter, randomized, double-blind LIGHT4LIFE pivotal clinical trial includes 108 patients, with mild to moderate stages, treated over a 6-month period. Results expected end of 2026. - Depression :
The preclinical study conducted at the University of Barcelona showed promising results for clinical translation. (Journal of Affective Disorders, 2023). - Multiple Sclerosis :
The preclinical study conducted at CNRS/Aix-Marseille University showed promising results for clinical translation (Journal of Neuroinflammation 2024). - Spinal Cord Injury :
The preclinical study at CNRS/Aix-Marseille University is currently underway.



